Biologic drugs have changed how we treat cancer, autoimmune diseases, and chronic conditions. But they come with a price tag that can hit $100,000 a year per patient. Enter biosimilars - drugs that are nearly identical to these expensive biologics, but cost far less. The question isn’t whether they work - they do. The real question is: why aren’t more people using them?
What Exactly Is a Biosimilar?
Biosimilars aren’t like generic pills. You can’t just swap one chemical compound for another. Biologics are made from living cells - proteins, antibodies, or other complex molecules grown in labs using bacteria or yeast. Because they’re alive, no two batches are exactly the same. That’s why a biosimilar isn’t a copy. It’s a very close match - so close that the FDA says there’s no meaningful difference in safety or effectiveness.
The first biosimilar got FDA approval in 2015. Since then, 30+ have been approved in the U.S., mostly for conditions like rheumatoid arthritis, Crohn’s disease, and cancer. But even though they’re approved, most patients are still getting the original biologic. Why? Because the pricing system doesn’t always reward savings.
How Much Do Biosimilars Actually Save?
It’s not 80% off like with generic pills. Biosimilars typically save between 15% and 35% off the list price of the original biologic. But that’s just the starting point.
Take Humira (adalimumab), the top-selling drug in the world for years. Before biosimilars hit the market in 2023, it cost around $7,000 per month. Now, with 10 FDA-approved biosimilars available - several of them labeled as “interchangeable” - the list price has dropped by up to 85%. That’s not a small discount. That’s life-changing for patients.
But here’s the catch: list price isn’t what most people pay. Drug manufacturers offer huge rebates to pharmacy benefit managers (PBMs) and insurers. These rebates are often tied to keeping the original drug on the formulary. So even if a biosimilar costs $1,000 a month and the original costs $7,000, the net price after rebates might be $5,000. That makes the biosimilar look less attractive - even though it’s still cheaper to produce and doesn’t need rebates to compete.
Real-world savings are clearer when you look at patient out-of-pocket costs. A 2025 study by CSRxP found that people using biosimilars paid 23% less on average than those on the original biologic. For some, that meant dropping from $500 a month to $385. For others, it meant going from $1,200 to $400. That’s not just savings - that’s access.
Why Don’t Biosimilars Save More?
The U.S. healthcare system is built to protect brand-name drugs, not reward competition. Here’s how it works:
- Biologic makers hold patents for 12 years, sometimes longer with legal tricks.
- When patents expire, they often sign deals with PBMs to keep their drug on top of formularies.
- These deals include rebates that can be as high as 70% of the drug’s list price.
- Biosimilars can’t match those rebates because they’re new and don’t have the same profit margins.
This is called the “rebate trap.” It’s why, even in 2025, originator biologics still make up 98.9% of all biologic spending in the U.S. That’s down from 99.6% in 2022 - progress, yes - but barely.
In Europe, where rebates are regulated and formularies are simpler, biosimilars make up 70-86% of the market for some drugs. In Norway, a biosimilar for Humira hit 86% market share within three years. In the U.S.? It’s still under 10%.
The Real Savings: Billions and Beyond
The numbers tell a bigger story. Since 2015, biosimilars have saved the U.S. healthcare system $56.2 billion. In 2024 alone, they saved $20.2 billion. That’s not pocket change. That’s enough to fund thousands of new cancer treatments, mental health programs, or diabetes screenings.
Employers are seeing it too. One analysis found that if every employee switched from a biologic to its biosimilar, an employer could save an average of $1.53 million per year. Across all self-insured U.S. companies, switching just two biologics to biosimilars could save $1.4 billion.
And the patient impact? Over 460 million extra days of therapy since 2015 - care that patients couldn’t afford before. That’s not a statistic. That’s someone getting to walk their child to school. That’s someone not having to choose between rent and their medication.
The Biosimilar Void: A $234 Billion Missed Opportunity
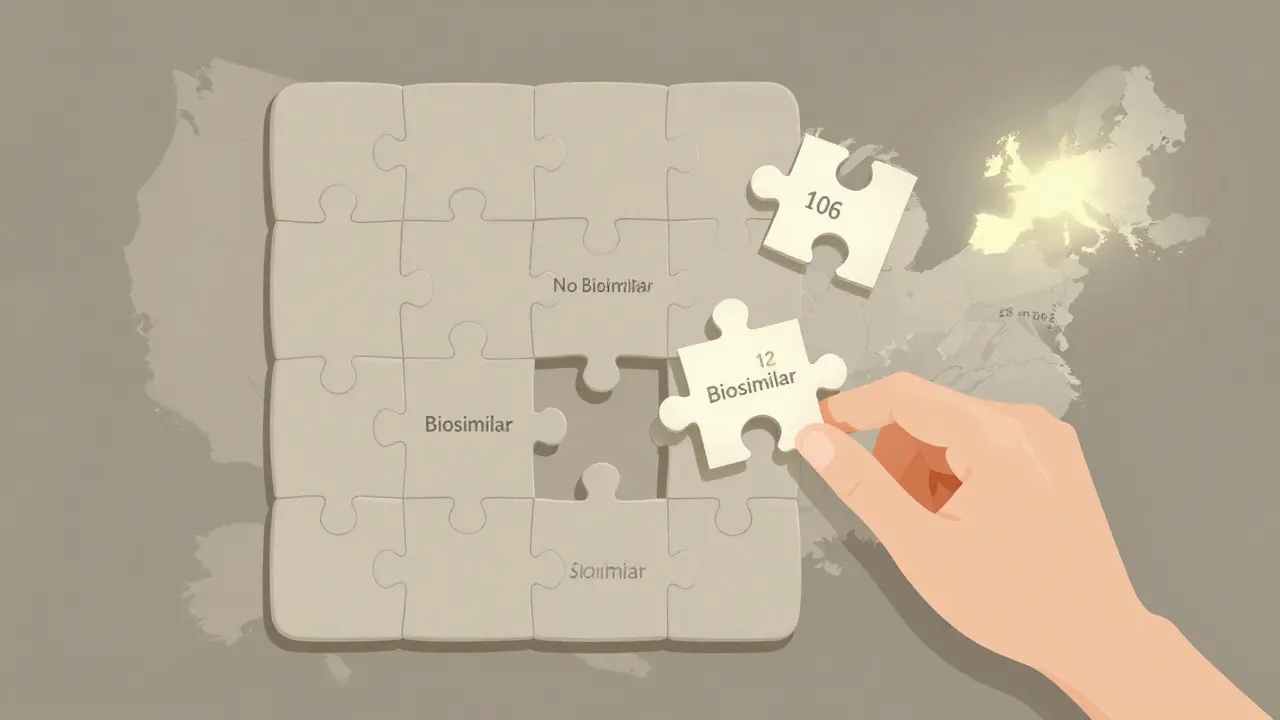
Here’s the scary part: 118 biologics are set to lose patent protection in the next 10 years. Only 12 of them have biosimilars in development. That means 90% of the next wave of expensive drugs won’t have cheaper alternatives.
That’s the “biosimilar void.” And it’s not just a problem for payers - it’s a crisis for patients. Without competition, prices will stay sky-high. The cost of treating cancer, psoriasis, or multiple sclerosis could keep rising, leaving millions locked out of care.
Europe doesn’t have this problem. They’ve had biosimilars for nearly 20 years. They’ve built a system that encourages competition. The U.S. is playing catch-up - and losing ground.
How to Get More Savings - and Why It Matters
Real change needs action from three places:
- Employers and insurers: Stop letting rebates dictate formulary decisions. Push for biosimilars as first-line options. Use step therapy: require patients to try the biosimilar before approving the original.
- Doctors and pharmacists: Educate patients. Many still think biosimilars are “inferior.” They’re not. They’re proven, safe, and just as effective.
- Policymakers: Limit rebates that block competition. Speed up FDA approvals. Fund biosimilar development for high-cost drugs with no competition.
It’s not complicated. The science is there. The savings are there. The patients are waiting.
What’s Next?
Stelara (ustekinumab), another top biologic, just saw nine biosimilars enter the market in July 2025. Prices are down as much as 90% off list. That’s a sign things are changing.
But change moves slowly. If nothing changes, the U.S. will miss out on $234 billion in potential savings over the next decade. That’s enough to cover the entire Medicare Part D program for two years.
The future of affordable biologic care isn’t about inventing new drugs. It’s about letting competition work. It’s about letting patients pay less. It’s about finally making the promise of biosimilars real.
Are biosimilars as safe and effective as the original biologics?
Yes. The FDA requires biosimilars to show no clinically meaningful differences in safety, purity, or potency compared to the original biologic. Over 460 million patient days of use since 2015 have shown no unique safety issues. Studies in JAMA Network Open and Frontiers in Pharmacology confirm they work just as well.
Why don’t biosimilars save 80-90% like generics?
Because biologics are made from living cells, not chemicals. Manufacturing them is complex, expensive, and requires years of testing. Generics are simple chemical copies - biosimilars are near-identical biological versions. That complexity means development costs are higher, so savings are more modest - usually 15-35% off list price, though sometimes much more when competition kicks in.
Can I switch from a biologic to a biosimilar safely?
Yes - especially if it’s labeled “interchangeable.” The FDA approves these as safe to swap without a doctor’s approval. Even non-interchangeable biosimilars have been used safely for years. Studies show no increased risk of side effects or loss of effectiveness when switching.
Why is my insurance still covering the original biologic instead of the cheaper biosimilar?
Many insurers and pharmacy benefit managers (PBMs) are locked into rebate deals with the original drug makers. Even if the biosimilar costs less, the rebate on the original can make its net price look competitive. This is called the “rebate trap.” You may need to ask your doctor or pharmacist to request a formulary exception.
Are biosimilars available for all biologic drugs?
No. Only about 12 of the 118 biologics set to lose patent protection in the next 10 years have biosimilars in development. Humira and Stelara have many options. But drugs for rare diseases or newer biologics often have none. That’s why experts call it the “biosimilar void.”
How do I know if a biosimilar is right for me?
Talk to your doctor. Ask if there’s an approved biosimilar for your condition. Check your insurance formulary. Ask if your pharmacy can fill it. Most biosimilars are covered under the same tier as the original, so your out-of-pocket cost may drop significantly - even if your plan hasn’t updated its rules yet.

Written by Felix Greendale
View all posts by: Felix Greendale