When you pick up a prescription, you might see a different name on the bottle than what your doctor wrote. That’s not a mistake - it’s a generic drug. Most people assume generics are just cheaper copies. But are they really the same? The answer isn’t simple, and it’s not just about price. Clinical studies over the last 30 years have dug deep into this question, and what they found surprises a lot of people.
What Does ‘Generic’ Actually Mean?
A generic drug isn’t a knockoff. It’s required by law to contain the exact same active ingredient, in the same strength, and delivered the same way as the brand-name version. That means if your doctor prescribes lisinopril for high blood pressure, the generic version has the same molecule doing the same job. The FDA doesn’t allow generics to be sold unless they meet this standard. But here’s where things get tricky: the inactive ingredients - the fillers, dyes, coatings - can be different. And for most people, that doesn’t matter. But for some, it can. Think of it like two different brands of aspirin. Both have acetylsalicylic acid. One might have a coating that dissolves slower. Another might have a flavoring that irritates your stomach. The active part? Identical. The experience? Sometimes not.How the FDA Proves Generics Work the Same
Before a generic drug hits the shelf, the manufacturer must prove it’s bioequivalent to the brand-name drug. That means in a study with 24 to 36 healthy volunteers, the body absorbs the generic drug at nearly the same rate and amount as the original. The FDA requires the blood concentration of the active ingredient to fall within 80% to 125% of the brand-name drug’s levels. That’s not a wide gap - it’s tight. For example, if the brand-name drug gives you a peak blood level of 100 units, the generic must land between 80 and 125. Most generics fall within 90-110%. This isn’t guesswork. It’s measured using precise tests called AUC (area under the curve) and Cmax (maximum concentration). A 2013 review of over 2,000 FDA-approved bioequivalence studies found no meaningful difference in how generics and brand-name drugs performed in the body. The FDA calls this the gold standard. And they don’t just approve one batch - they inspect manufacturing sites and test multiple batches over time.What Do Real-World Studies Say?
Lab tests are one thing. Real patients are another. So researchers looked at millions of people taking generics in everyday life. A massive 2019 study by R.J. Desai, tracking 3.5 million patients, found no difference in outcomes between generics and brand-name drugs for common conditions like high blood pressure (amlodipine), diabetes (glipizide), osteoporosis (alendronate), and depression (escitalopram). In fact, for amlodipine, patients on generics had slightly lower risk of heart attacks and hospitalizations. Even more striking was a 2020 study in Austria that followed 1.2 million people with chronic illnesses over five years. The results? People taking generics had fewer deaths and fewer major heart events than those on brand-name versions. Why? Researchers think it might be because generics are cheaper, so people take them consistently. Missing doses because of cost is a real problem - and generics fix that.When Generics Don’t Work - The Exceptions
But here’s the part no one talks about enough: sometimes, they don’t. Drugs with a narrow therapeutic index are the exception. These are medications where even a tiny change in blood level can cause big problems. Think seizure meds like levetiracetam or lamotrigine, blood thinners like warfarin, thyroid meds like levothyroxine, and some epilepsy drugs. A 2023 study in Epilepsia found that switching between different generic versions of levetiracetam led to an 18% higher chance of seizures. Patients on levothyroxine have reported wild swings in thyroid levels after switching generic brands - even when both were labeled as “bioequivalent.” One Reddit user wrote: “I tried three different generics for Synthroid. Only one kept my TSH stable.” In rare cases, patients report side effects or loss of effectiveness after switching. A 2013 study found that 30% of patients saw no change, 30% felt worse, and 30% stopped taking the drug altogether after switching to a generic. These aren’t random. They’re real people whose lives were disrupted by a change they didn’t ask for.Why Do These Differences Happen?
It’s not fraud. It’s science. Even if two generics have the same active ingredient, differences in how they’re made - particle size, coating, dissolution rate - can change how fast the drug enters your bloodstream. For most drugs, that doesn’t matter. For thyroid or epilepsy meds, it can. Also, some generics are made by different companies overseas. Quality control varies. The 2021 valsartan recall affected multiple generic brands because of cancer-causing impurities. That wasn’t about efficacy - it was about safety. But it eroded trust. And then there’s the placebo effect - but backwards. If you believe generics are inferior, your body might react as if they are. Studies show patients who know they’re taking a generic report more side effects, even when the drug is identical to the brand.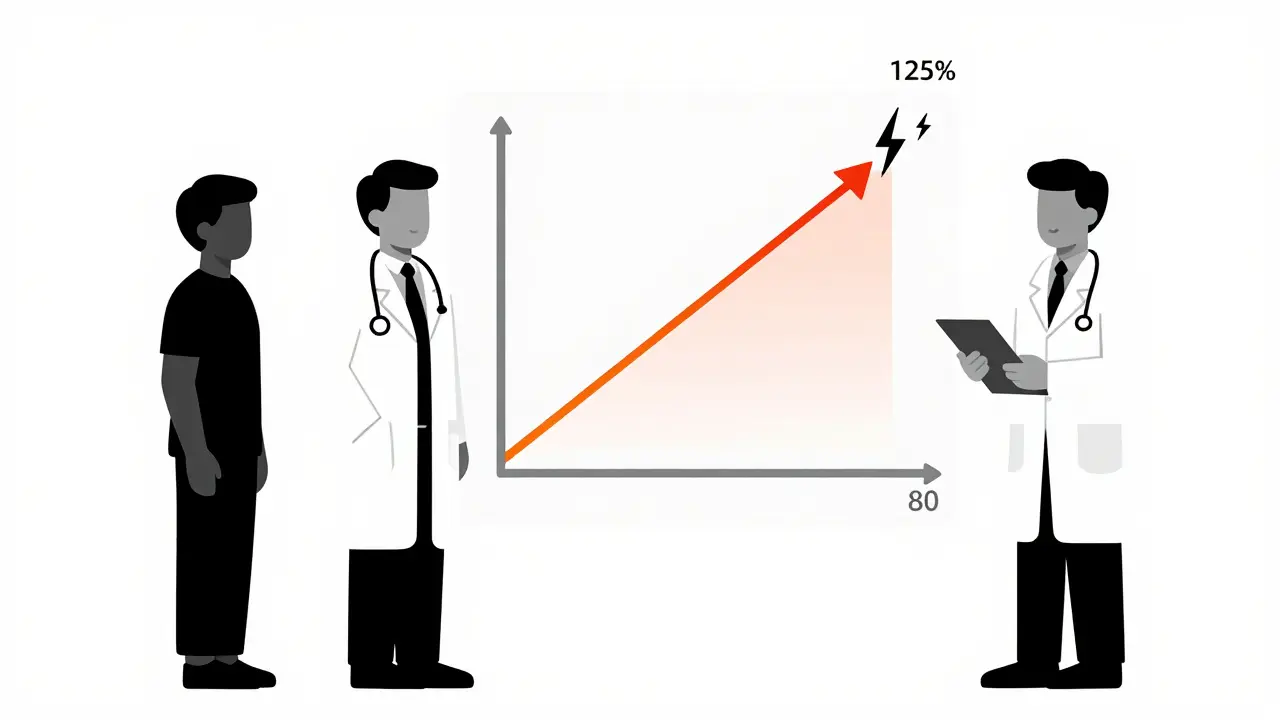
What Doctors and Pharmacists Know
Most doctors trust generics. A 2020 survey found 87% of physicians have confidence in their effectiveness. But specialists - neurologists, endocrinologists, cardiologists - are more cautious. They’ve seen the outliers. Pharmacists are trained to substitute generics unless the doctor writes “dispense as written.” That happens in only 3.2% of cases. But they also hear the complaints. A 2021 survey found 42% of pharmacists say patients worry about generic quality - not because they’re wrong, but because they’ve been burned before. The FDA’s Orange Book lists which generics are rated “A” (therapeutically equivalent) and which are “B” (potential differences). But most patients never see this. Pharmacy websites focus on price, not science.What You Should Do
If you’re taking a generic drug and it’s working - don’t change a thing. You’re saving money and getting the same result. But if you’re on a narrow therapeutic index drug - thyroid, epilepsy, warfarin, digoxin, lithium - pay attention. If you notice new side effects, changes in how you feel, or lab results that don’t match, talk to your doctor. Ask: “Could this be the generic?” Don’t assume all generics are the same. If one brand works, stick with it. You can ask your pharmacist to keep giving you the same manufacturer. Some pharmacies will honor that. If your insurance switches your generic without warning, and you feel worse - push back. You have the right to request the brand or a specific generic. Just say: “I need to stay on this version because it works for me.”The Bigger Picture
Generics saved the U.S. healthcare system $377 billion a year. Without them, millions couldn’t afford their meds. They’re not just a cost-cutting tool - they’re a lifeline. But they’re not perfect. The system works for 99% of people. For the 1% - the ones with epilepsy, thyroid disease, or heart conditions - the stakes are higher. We need better testing for complex generics. We need clearer labeling. And we need doctors and pharmacists to listen when patients say, “This one doesn’t feel right.” The science says generics work. But real life is messier than a lab report. The best answer isn’t “all generics are equal.” It’s: “Know your drug. Know your body. And don’t be afraid to speak up.”Are generic drugs really as effective as brand-name drugs?
For most medications, yes. The FDA requires generics to prove they deliver the same amount of active ingredient into your bloodstream at the same rate as the brand-name version. Large studies involving millions of patients show no difference in outcomes for common drugs like blood pressure pills, antidepressants, and diabetes meds. But for drugs with a narrow therapeutic index - like thyroid medicine, seizure drugs, or blood thinners - small differences can matter. Some patients report changes in how they feel after switching generics.
Why do some people say generics don’t work for them?
There are a few reasons. First, inactive ingredients (fillers, coatings) can affect how fast the drug dissolves - which matters most for drugs where blood levels must stay very tight. Second, switching between different generic manufacturers can cause changes, even if both are “bioequivalent.” Third, psychological factors play a role - if you believe generics are inferior, you may notice side effects more. And finally, rare manufacturing issues (like impurities) can happen, though they’re not common.
Can I ask my pharmacist to give me the same generic brand every time?
Yes. While pharmacists are allowed to substitute generics, you have the right to request a specific manufacturer. Tell them: “I need to stay on this version because it works for me.” Many pharmacies will honor that request, especially if you’ve had issues switching before. You can also ask your doctor to write “dispense as written” or “do not substitute” on your prescription.
Are generic drugs made in the same facilities as brand-name drugs?
Sometimes. Many brand-name companies also make generic versions of their own drugs. Other generics are made by independent manufacturers, often overseas. The FDA inspects all facilities - whether they make brand or generic drugs - and holds them to the same quality standards. But inspections don’t catch every issue. That’s why recalls happen. The key is that the FDA requires all manufacturers to meet the same strict standards, regardless of where they’re based.
Should I avoid generics if I have a serious health condition?
No - but be smart. For most conditions, generics are safe and effective. But if you’re on a drug with a narrow therapeutic index - like levothyroxine, warfarin, or seizure medications - monitor how you feel after a switch. Track your symptoms and lab results. If something changes, tell your doctor. Don’t assume the generic is the problem - but don’t ignore it either. Your health is worth the extra attention.
How do I know if my generic is FDA-approved and safe?
All legally sold generics in the U.S. must be FDA-approved. You can check the FDA’s Orange Book online to see if your drug is rated “A” (therapeutically equivalent). But you don’t need to do that yourself. Your pharmacist knows. If you’re worried, ask them: “Is this generic FDA-approved and rated A?” If they hesitate or can’t answer, that’s a red flag. Legitimate generics come with full FDA oversight.

Written by Felix Greendale
View all posts by: Felix Greendale