Switching to generic medications can cut your monthly drug bill by 90% - but only if you know how to do it right. You’ve probably heard the rumors: generics are cheaper, but are they safe? Do they work the same? What if your blood pressure spikes after switching? These aren’t just fears - they’re real concerns, backed by data. The truth is, for most people, generics are just as safe and effective as brand-name drugs. But there are exceptions. And knowing where those exceptions lie can save you from hospital visits, emergency room trips, and wasted money.
What Exactly Is a Generic Drug?
A generic drug isn’t a copy. It’s the same medicine. The FDA requires that generics contain the exact same active ingredient, strength, dosage form, and route of administration as the brand-name version. That means if you take a generic version of metformin, you’re getting the same chemical that’s in Glucophage. The same goes for lisinopril, atorvastatin, or levothyroxine. The only differences are in the inactive ingredients - the fillers, dyes, or flavors that don’t affect how the drug works. A generic pill might be white instead of blue, oval instead of round, and have a different imprint. But the part that treats your condition? Identical.How Much Money Can You Really Save?
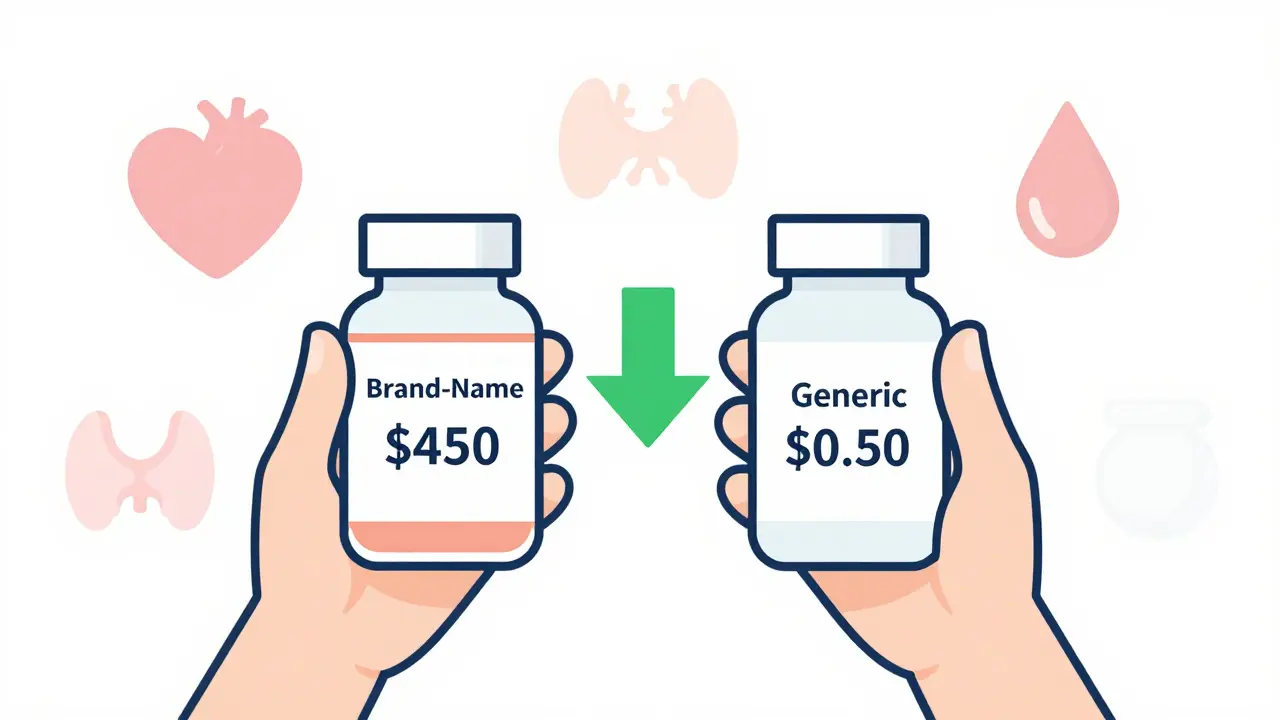
The numbers speak for themselves. A 30-day supply of brand-name Lipitor (atorvastatin) costs around $450. The generic? As low as $0.50 at major U.S. pharmacies. Plavix (clopidogrel) runs $380 a month. Generic clopidogrel? $1.20. Even for more common prescriptions like amoxicillin or metformin, the savings are dramatic. The average generic prescription costs $14.50. The average brand-name? Over $620. Since 2009, generics have saved the U.S. healthcare system nearly $2 trillion. If you’re on even one chronic medication, switching to generic could save you $500 to $1,500 a year. That’s a vacation. A new tire. A month’s rent.When Are Generics Just as Safe?
For most drugs - and most people - generics are just as safe. A 2020 study in Nature Communications looked at 17 cardiovascular drugs and found that generic versions were linked to fewer deaths in 10 of them. Another study tracking over 2 million patients in 2024 found that when patients stayed on the same generic manufacturer, their risk of heart attack or stroke was no different than those on brand-name statins. The FDA has reviewed over 12,000 generic applications since 2022 and found no evidence that generics are less safe. In fact, the same manufacturing standards apply. Generic factories are inspected just as often as brand-name ones - around 1,800 inspections a year - and must meet the same strict rules for quality, purity, and stability.The Exceptions: When You Need to Be Careful
Not all drugs are created equal when it comes to switching. Some have what’s called a narrow therapeutic index - meaning the difference between a dose that works and one that’s dangerous is tiny. For these, even small changes in how the drug is absorbed can matter. The big ones to watch:- Warfarin (blood thinner): Switching generics can cause INR levels to fluctuate, increasing risk of clots or bleeding.
- Levothyroxine (thyroid hormone): Studies show 23% of patients on generic versions report inconsistent symptoms compared to 8% on Synthroid. Thyroid levels are sensitive - even small changes can cause fatigue, weight gain, or heart palpitations.
- Phenytoin (anti-seizure): Multiple switches between generic manufacturers have been linked to higher seizure recurrence rates - up to 12% in some studies.
- Lithium (bipolar disorder): Small changes in blood levels can trigger mood episodes or toxicity.
What About That One Study Saying Generic Statins Are Riskier?
Yes, a 2023 meta-analysis suggested a higher risk of major cardiac events with some generic statins. But here’s the catch: that study didn’t control for manufacturer consistency. Later research in 2024 tracked patients who stayed on the same generic statin brand and found no increased risk. The real issue? Switching between different generic manufacturers - not generics themselves. If you’re on a generic statin and your pharmacy changes the maker without telling you, your body might react. That’s not the drug’s fault - it’s a lack of communication.How to Switch Safely (The 3 Questions to Ask Your Pharmacist)
You don’t need to be a pharmacist to protect yourself. Just ask these three questions every time you fill a prescription:- Is this generic from an FDA-inspected facility? All U.S. generics are, but many are made overseas. You can check the FDA’s Drugs@FDA database to see where your drug is made.
- Are there multiple generic makers for this drug? If yes, that means competition - and lower prices. But it also means you might get a different version each time. Ask if you can stick with one brand.
- Is this a narrow therapeutic index drug? If your doctor says yes, don’t switch without a blood test. For drugs like warfarin or levothyroxine, your doctor should monitor your levels after any switch.
What to Do If You Feel Different After Switching
If you notice new side effects - dizziness, fatigue, nausea, mood changes - after switching to a generic, don’t assume it’s all in your head. Track it. Write down when it started, what you’re feeling, and how it compares to before. Then go back to your pharmacist or doctor. Sometimes, it’s just your body adjusting. Other times, it’s a reaction to a different filler. If you’re on a high-risk drug, ask for a blood test. For thyroid or blood thinners, even a small change in lab values can signal a problem.How to Stay Consistent
Once you find a generic that works, keep it. Check the pill’s color, shape, and imprint each time you refill. If it looks different, ask why. The FDA’s Orange Book lists all approved generics and their manufacturers. You can look up your drug and see which companies make it. If your pharmacy switches manufacturers without warning, speak up. You have the right to request a specific brand. Many pharmacies will honor that - especially if you explain you’re on a narrow therapeutic index drug.Why Pharmacists Are Your Secret Weapon
A 2023 University of Michigan study found that patients who got structured counseling from pharmacists about generics had 32% higher adherence and 27% fewer medication problems. Pharmacists see every prescription, every switch, every complaint. They know which generics have had issues, which manufacturers are reliable, and which ones cause more side effects. Don’t just pick up your script and leave. Take two minutes to talk to them. Ask: “Have you seen any problems with this generic?” Most will be happy to help.What’s Next? Biosimilars and the Future
Generics aren’t just for pills anymore. Biosimilars - generic versions of complex biologic drugs like Humira or Enbrel - are now approved for cancer, arthritis, and autoimmune diseases. These aren’t simple copies; they’re highly similar versions made from living cells. The FDA requires them to meet the same safety and effectiveness standards as the original. And like traditional generics, they cost 15-35% less. By 2030, biosimilars could save the U.S. system $300 billion. The same principles apply: know your drug, ask questions, and don’t switch without monitoring.Bottom Line: Generics Are Safe - If You Play Smart
For 9 out of 10 people, switching to generic medication is a no-brainer. You get the same results, at a fraction of the cost. But for the 1 in 10 on narrow therapeutic index drugs, or those who’ve had bad reactions before, caution is key. Don’t let fear stop you from saving money. But don’t let convenience override safety. Know your drug. Know your manufacturer. Know your body. And talk to your pharmacist. The savings are real. The risks are manageable. And with the right approach, you can stay healthy - and keep more money in your pocket.Are generic drugs as effective as brand-name drugs?
Yes, for most drugs, generics are just as effective. The FDA requires them to have the same active ingredient, strength, and absorption rate as the brand-name version. Studies show identical outcomes for drugs like blood pressure meds, antibiotics, and antidepressants. The only exceptions are narrow therapeutic index drugs like warfarin, levothyroxine, and phenytoin, where small changes in absorption can affect safety.
Why do some people feel worse on generic medications?
Most often, it’s not the active ingredient - it’s the inactive ones. Different fillers, dyes, or coatings can cause minor side effects like stomach upset or headaches. Sometimes, switching between generic manufacturers changes the pill’s absorption slightly, especially for sensitive drugs. A 2012 study found 10% of patients reported side effects after switching, but most resolved within two weeks. If symptoms persist, ask your doctor for a blood test or to lock in one manufacturer.
Can I ask my pharmacist to give me the same generic every time?
Yes. While pharmacies often choose the cheapest option, you have the right to request a specific generic manufacturer. This is especially important for drugs with narrow therapeutic windows. Tell your pharmacist you want to stay on the same brand to avoid fluctuations. Many will accommodate you, especially if you explain your health concerns.
How do I know if my generic is made in a safe facility?
All generics sold in the U.S. must come from FDA-inspected facilities - whether in the U.S., India, or elsewhere. You can verify this by searching your drug in the FDA’s Drugs@FDA database. Look for the manufacturer name and check if it’s listed as approved. If your pharmacy switches to a new maker you don’t recognize, ask them to confirm it’s FDA-approved.
Should I avoid generics if I have epilepsy or thyroid disease?
Not necessarily - but be cautious. For epilepsy, multiple switches between generic antiepileptic drugs have been linked to higher seizure rates. For thyroid disease, generic levothyroxine can cause inconsistent symptom control in some people. The best approach is to pick one generic manufacturer and stick with it. Never switch without consulting your doctor and getting a blood test to check your levels.
Do generics take longer to work than brand-name drugs?
No. The FDA requires generics to be bioequivalent - meaning they’re absorbed into your bloodstream at the same rate and to the same extent as the brand-name version. For most drugs, you’ll feel the effects at the same time. If you notice a delay, it could be due to a change in manufacturer or inactive ingredients, not the active drug itself.
Is it safe to switch from brand-name to generic mid-treatment?
For most medications, yes. But for drugs like warfarin, lithium, or levothyroxine, it’s risky without monitoring. Always talk to your doctor first. If you do switch, schedule a follow-up blood test within 2-4 weeks. For non-critical drugs like antibiotics or statins, switching mid-treatment is generally safe and often encouraged to reduce costs.

Written by Felix Greendale
View all posts by: Felix Greendale