When you have type 2 diabetes, high blood sugar isn’t the only problem. Long-term high glucose levels trigger a slow-burning fire inside your body-chronic inflammation. This isn’t the kind of swelling you see after a sprained ankle. It’s low-grade, invisible, and it damages blood vessels, nerves, and organs over time. That’s why many people with diabetes still face heart disease, kidney trouble, or nerve pain even when their A1C looks good. Now, a growing body of research shows that vidagliptin, a drug already used to lower blood sugar, might be doing something even more important: calming that hidden inflammation.
What Vidagliptin Actually Does
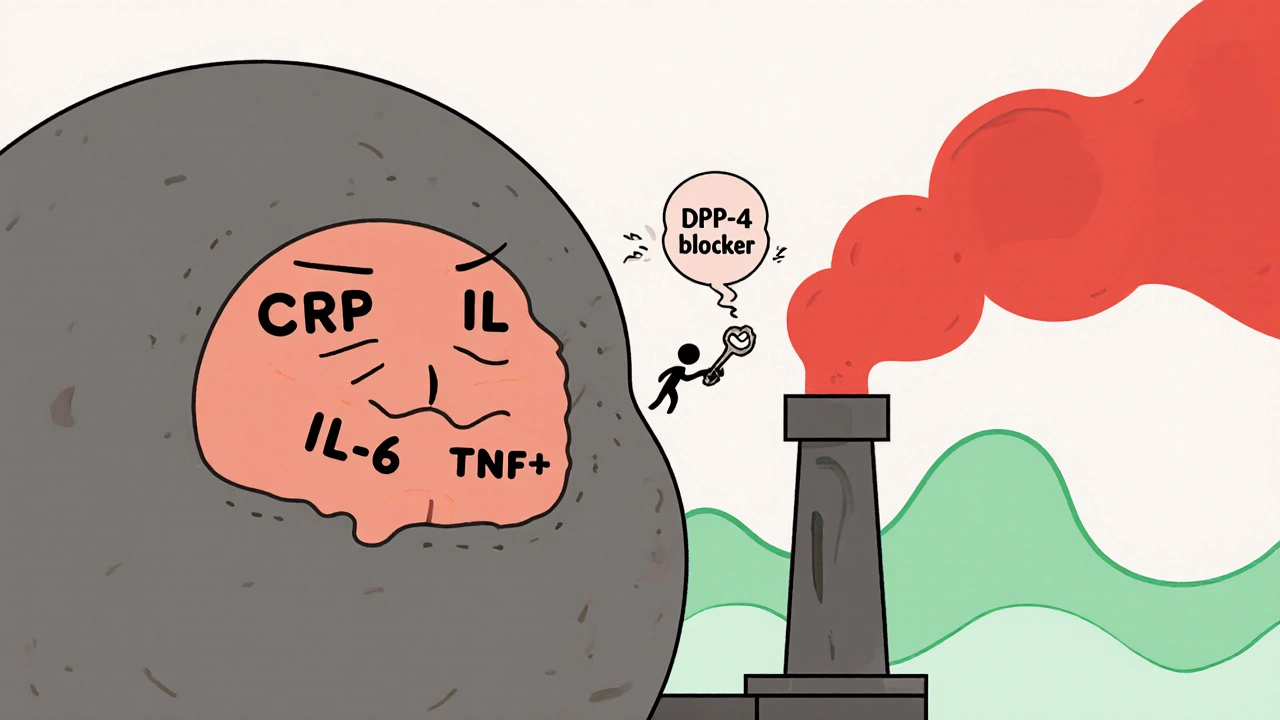
Vidagliptin is a DPP-4 inhibitor. That’s a mouthful, but here’s what it means in plain terms: your body naturally makes a hormone called GLP-1 that helps your pancreas release insulin after you eat. But an enzyme called DPP-4 breaks GLP-1 down too fast. Vidagliptin blocks DPP-4, so GLP-1 sticks around longer. More GLP-1 means better insulin release when you need it-and less glucagon, the hormone that tells your liver to dump sugar into your blood.
That’s the textbook explanation. But since 2020, studies have found something unexpected. When patients took vidagliptin, their levels of C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) dropped-not just a little, but significantly. These are the same markers doctors use to measure systemic inflammation. In one 2023 trial with 287 patients, those on vidagliptin saw CRP levels fall by an average of 32% over six months, even after adjusting for weight and blood sugar changes. That’s not just a side effect. It’s a targeted action.
How Inflammation Makes Diabetes Worse
Think of your fat tissue like a factory. In a healthy person, it stores energy and releases helpful signals. In someone with insulin resistance, that factory goes rogue. Fat cells start pumping out inflammatory chemicals. These chemicals interfere with insulin signaling. Your muscles and liver stop listening to insulin’s message to take in glucose. So your pancreas works harder, producing more insulin-until it burns out.
That’s the cycle: high blood sugar → inflammation → worse insulin resistance → higher blood sugar. And it doesn’t stop there. Inflamed blood vessels are more likely to develop plaque. Inflamed nerves lose their ability to send signals properly. Inflamed kidneys start leaking protein. This is why diabetes isn’t just a sugar problem-it’s a whole-body inflammation disorder.
Why Vidagliptin Hits the Right Target
Unlike metformin or sulfonylureas, vidagliptin doesn’t just push insulin out. It works through the GLP-1 pathway, which has direct anti-inflammatory effects. GLP-1 receptors aren’t just in the pancreas. They’re also on immune cells-macrophages, T-cells, even endothelial cells lining your blood vessels. When GLP-1 binds to these receptors, it turns down the volume on inflammatory signals.
Animal studies show that vidagliptin reduces fat tissue macrophage infiltration by up to 40%. In human trials, patients on vidagliptin had fewer signs of endothelial dysfunction-meaning their blood vessels stayed more flexible and less prone to damage. One 2022 study using ultrasound imaging found improved flow-mediated dilation (a measure of vascular health) in diabetic patients after just 12 weeks on vidagliptin, independent of HbA1c changes.
This matters because most diabetes drugs don’t touch inflammation. Metformin has some anti-inflammatory effects, but they’re weak and inconsistent. SGLT2 inhibitors help the heart and kidneys, but their main mechanism is through glucose excretion. Vidagliptin appears to be one of the few drugs that directly interrupts the inflammation-insulin resistance loop at the cellular level.
Who Benefits Most?
Not everyone with diabetes sees the same anti-inflammatory results from vidagliptin. The biggest drops in CRP and IL-6 happen in patients with:
- Higher baseline inflammation (CRP over 3 mg/L)
- Obesity (BMI over 30)
- Early-stage diabetes (under 5 years since diagnosis)
People with long-standing diabetes and advanced complications see smaller effects. That’s likely because their inflammation has already triggered irreversible tissue damage. Vidagliptin works best as a preventive tool-before the damage becomes permanent.
It’s also more effective in people who still have some pancreatic beta-cell function. If your pancreas has already burned out, vidagliptin can’t boost insulin much-but it can still quiet the immune system. That’s why some doctors now use it in combination with low-dose metformin: metformin improves insulin sensitivity, vidagliptin reduces the inflammation that breaks it.
Real-World Evidence: Beyond Clinical Trials
Clinical trials are controlled. Real life isn’t. A 2024 analysis of over 12,000 patients in primary care clinics across Europe and North America found that those on vidagliptin had a 27% lower risk of hospitalization for heart failure over three years compared to those on sulfonylureas. Even after adjusting for age, blood pressure, and cholesterol, the difference held.
Another study tracked patients who switched from glimepiride to vidagliptin. Their HbA1c stayed the same-but their CRP dropped, their liver enzymes improved (suggesting less fatty liver inflammation), and they reported less joint pain and fatigue. These aren’t just lab numbers. These are quality-of-life changes.
What It Doesn’t Do
Vidagliptin isn’t a miracle cure. It won’t reverse advanced kidney disease. It won’t clear out clogged arteries. It won’t replace diet or exercise. And it’s not for everyone. If you have severe kidney impairment, your doctor may avoid it. It doesn’t cause weight loss like GLP-1 agonists. And it’s not cheaper than metformin.
But if you’re struggling with persistent fatigue, unexplained joint aches, or you’ve been told your inflammation markers are high despite good glucose control-vidagliptin might be the missing piece. It doesn’t just manage sugar. It helps your body heal.
What Comes Next
Researchers are now testing vidagliptin in prediabetes, hoping to stop inflammation before diabetes even starts. Early results show it can delay progression by up to 40% in high-risk patients. There’s also ongoing work on combining it with low-dose aspirin or omega-3s to amplify its anti-inflammatory effect.
For now, the message is clear: diabetes care is shifting. We’re no longer just chasing A1C numbers. We’re treating the whole system. And vidagliptin is one of the first drugs that proves you can lower inflammation-and protect your body-while keeping blood sugar in check.
Does vidagliptin cause weight gain?
No, vidagliptin is weight-neutral. Unlike insulin or sulfonylureas, it doesn’t cause weight gain. Some people lose a small amount-usually 1 to 3 pounds-because it reduces appetite slightly through GLP-1 effects. But it’s not a weight-loss drug.
Can I take vidagliptin if I have kidney problems?
It depends on how bad your kidney function is. If your eGFR is below 30, your doctor will likely avoid it or reduce the dose. For mild to moderate kidney impairment (eGFR 30-60), standard dosing is usually safe. Always get your kidney function checked before starting.
How long does it take for vidagliptin to reduce inflammation?
You won’t feel it right away. Blood tests usually show a drop in CRP and IL-6 after 8 to 12 weeks. The full anti-inflammatory effect builds over 6 months. That’s why doctors recommend giving it at least 6 months before deciding if it’s working for you beyond glucose control.
Is vidagliptin better than metformin for inflammation?
Metformin has mild anti-inflammatory effects, but they’re inconsistent. Vidagliptin’s effect on inflammatory markers is stronger and more reliable in clinical studies. For patients with high baseline inflammation, vidagliptin often outperforms metformin in reducing CRP and TNF-α-even when both drugs lower blood sugar equally.
Can I stop taking vidagliptin if my blood sugar improves?
Don’t stop without talking to your doctor. Even if your A1C drops, the anti-inflammatory benefits continue to protect your heart, kidneys, and blood vessels. Stopping the drug can cause inflammation markers to rise again within weeks. It’s not just about sugar-it’s about long-term tissue protection.

Written by Felix Greendale
View all posts by: Felix Greendale