Switching from brand-name to generic immunosuppressants like cyclosporine and tacrolimus can save thousands of dollars a year. But for transplant patients, that savings comes with a hidden risk: tiny differences in how the body absorbs the drug can trigger rejection, toxicity, or hospitalization. These aren’t just any generics-they’re narrow therapeutic index (NTI) drugs, where even a 10% change in blood levels can mean the difference between survival and organ failure.
Why These Two Drugs Are So Dangerous to Switch
Cyclosporine and tacrolimus both block the same immune pathway-calcineurin-but they do it in completely different ways. Cyclosporine binds to cyclophilin; tacrolimus binds to FKBP proteins. Both stop T-cells from attacking the new organ. But here’s the catch: tacrolimus works at 20 to 100 times lower doses than cyclosporine. A typical daily dose of tacrolimus is around 5 mg twice a day. Cyclosporine? Around 150 mg twice a day. That’s not just a difference in strength-it’s a difference in how the body handles every milligram. Therapeutic ranges are razor-thin. For tacrolimus, doctors aim for 5-15 ng/mL in the blood during the first six months after transplant. Go below 5, and rejection kicks in. Go above 15, and you risk kidney damage, tremors, or even seizures. Cyclosporine’s target is 100-200 ng/mL, but even within that range, small shifts matter. A patient on 150 mg of cyclosporine might see their level drop from 180 to 140 ng/mL after switching generics-and that’s enough to trigger acute rejection.Generic Versions Aren’t All the Same
There are 14 FDA-approved generic versions of tacrolimus and 11 of cyclosporine. Each one is made by a different company: Mylan, Teva, Apotex, Sandoz, and others. The FDA says they’re bioequivalent if blood levels fall within 80-125% of the brand-name drug. Sounds fair, right? But that 45% window is huge for NTI drugs. A 2022 study in Clinical Transplantation found that 73% of transplant centers saw blood level changes when patients switched between different generic manufacturers-even if both were labeled as “tacrolimus.” One patient switched from Generic A to Generic B, both approved by the FDA. Their tacrolimus level dropped from 8.5 ng/mL to 5.2 ng/mL in two weeks. They ended up in the hospital with a mild rejection episode. That’s not rare. A 2022 survey of 1,247 transplant patients found 42.7% noticed new side effects after switching generics. Nearly one in five needed a dose adjustment. Cyclosporine is even trickier. Older versions used oil-based capsules that absorbed inconsistently. The newer microemulsion version (Neoral) fixed that-but generic versions still vary in how they’re formulated. Some use different oils, fillers, or coatings. One patient reported stable levels on Generic C for a year. Switched to Generic D, and their levels spiked overnight. Their nephrologist had to reduce the dose by 30% to avoid toxicity.Real Stories, Real Consequences
Reddit’s r/transplant community has hundreds of threads like this:- “Switched from Prograf to generic tacrolimus. My levels crashed. Rejection. Hospitalized.” - u/KidneyWarrior, March 2023
- “My insurance forced me to switch. I’ve been on the same generic for 18 months now. Stable. Saved $900/month.” - u/TransplantSurvivor
- “My doctor won’t let me switch to generic cyclosporine. Last time we tried, my levels bounced like a ping-pong ball.” - u/OrganRecipient99
How Transplant Centers Are Fighting Back
Hospitals aren’t ignoring this. Most now have strict rules:- Never switch between generic brands without checking blood levels.
- Monitor levels weekly for at least four weeks after any switch.
- Stick to one generic manufacturer if possible. Many centers now sign “single-source” contracts with pharmacies to ensure consistency.
- Require pharmacists to document the exact generic name and manufacturer on every prescription.
What Patients Can Do
You don’t have to accept random switches. Here’s what works:- Ask your pharmacist: “Which generic brand am I getting?” Write it down. If it changes, ask why.
- Insist on blood tests after any switch-even if your doctor says it’s “not necessary.”
- Avoid grapefruit, pomegranate, and St. John’s wort. They interfere with how your body breaks down both drugs.
- Take your dose at the same time every day, within one hour. Even a 2-hour delay can cause levels to dip.
- If you feel new tremors, headaches, or fatigue after a switch, get your levels checked immediately.
The Future: Better Solutions
In December 2023, Astellas got FDA approval for a new extended-release version of tacrolimus called LCP-tacrolimus. It’s designed to release the drug slowly, smoothing out those dangerous peaks and valleys. Early data shows fewer fluctuations-meaning fewer switches between generics might be needed. The European Medicines Agency now requires generic manufacturers to test their products in actual transplant patients, not just healthy volunteers. That’s a big step. The U.S. might follow. Long-term, genetic testing is changing the game. About 20% of people carry a gene variant (CYP3A5*1) that breaks down tacrolimus faster. These patients need higher doses. A 2023 JAMA Internal Medicine study showed that dosing based on CYP3A5 genotype cut the time to reach stable levels by 63%. Imagine avoiding the guesswork entirely.Bottom Line: Savings Shouldn’t Cost Your Organ
Generic cyclosporine and tacrolimus save patients and insurers thousands. That’s good. But these aren’t antibiotics or blood pressure pills. They’re life-or-death drugs with no room for error. A 20% drop in blood levels might mean a 50% higher risk of rejection. If you’re on one of these drugs, don’t let cost be the only decision. Ask questions. Track your levels. Know your generic. Stay consistent. Your new organ didn’t come with a warranty. But you can still protect it.Can I switch between different generic versions of tacrolimus without checking my blood levels?
No. Switching between different generic manufacturers of tacrolimus-even if both are FDA-approved-can cause dangerous shifts in blood levels. Transplant centers require weekly blood tests for at least four weeks after any switch. Skipping this step risks rejection or toxicity.
Why is tacrolimus more dangerous than cyclosporine when switching generics?
Tacrolimus has a narrower therapeutic range (5-15 ng/mL) compared to cyclosporine (100-200 ng/mL). Small changes in absorption matter more because the difference between a safe and toxic dose is smaller. Also, tacrolimus is metabolized more unpredictably across individuals, making it more sensitive to formulation differences.
Is brand-name Prograf or Neoral still worth the cost?
For some patients, yes. If you’ve been stable on brand-name for years and switching causes level fluctuations, staying on brand may be safer. Many insurance plans now require prior authorization for brand-name drugs, but if your doctor documents instability with generics, they can often approve the brand. The cost difference is significant-$1,200/month vs. $300-but so is the risk of rejection.
What should I do if my pharmacy switches my generic without telling me?
Call your transplant center immediately. Request a blood level check within 48 hours. Then contact your pharmacist and insist on being notified before any future switches. You have the right to know which generic you’re getting. If they refuse, ask for a different pharmacy or file a complaint with your state board of pharmacy.
Are there any new treatments that reduce the risk of generic switching?
Yes. A new extended-release tacrolimus (LCP-tacrolimus) was approved in late 2023 and reduces blood level swings. Also, genetic testing for CYP3A5 can help doctors start you on the right dose from day one, reducing the need for frequent adjustments. These tools make generic use safer-but they’re not yet standard everywhere.
How can I find out which generic manufacturer I’m on?
Check the pill bottle label-it should list the manufacturer. If it doesn’t, ask your pharmacist for the name and lot number. Write it down. Keep a record of every switch. If you’re on Medicare Part D, your plan’s formulary list may show approved generics, but it won’t tell you which one you’re getting. You have to ask.

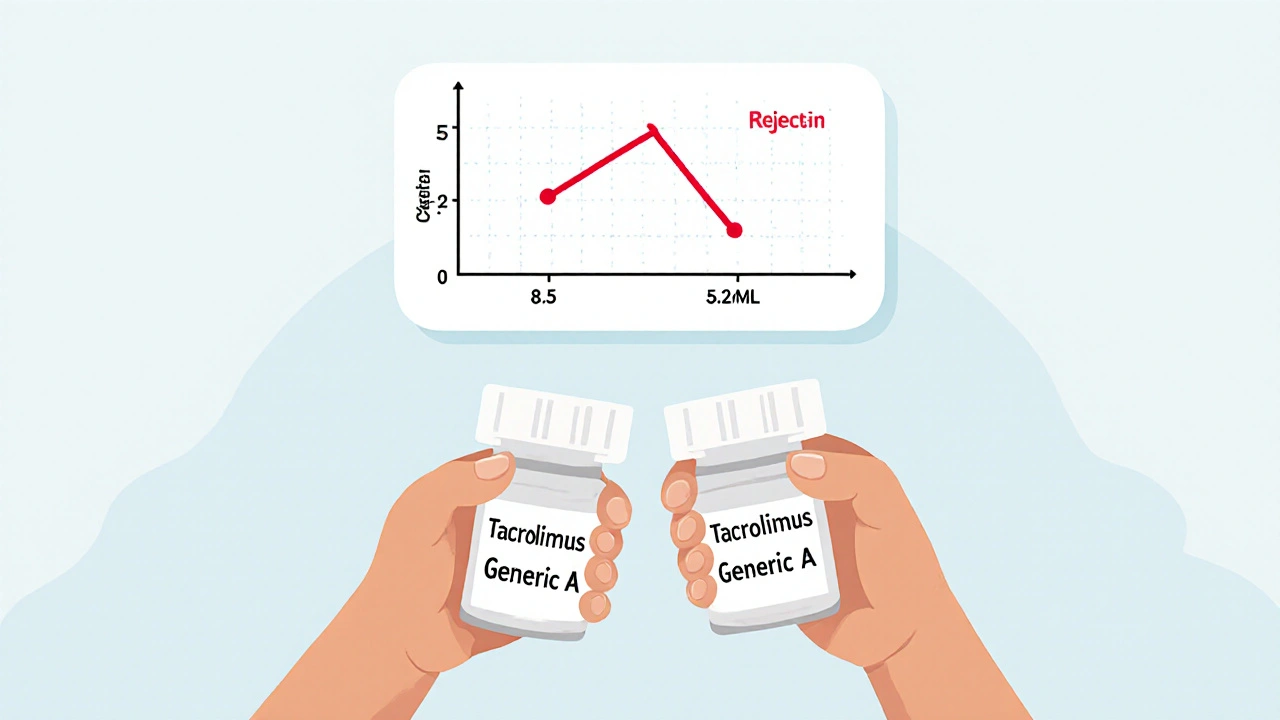
Written by Felix Greendale
View all posts by: Felix Greendale