By 2025, hepatitis C is no longer the slow killer it once was. A single pill, taken for 8 to 12 weeks, can cure more than 95% of people - and sofosbuvir is at the heart of that revolution. But it’s not the only option. Doctors now have a whole toolbox of direct-acting antivirals (DAAs), each with different strengths, side effects, and costs. So when you’re choosing a treatment, what does sofosbuvir really offer compared to the rest?
What is sofosbuvir, really?
Sofosbuvir is a nucleotide analog polymerase inhibitor that blocks the hepatitis C virus from copying its genetic material. It was first approved by the FDA in 2013 and quickly became the backbone of hepatitis C treatment worldwide. Unlike older interferon-based therapies that caused severe flu-like symptoms and required injections, sofosbuvir is taken as a daily tablet. It works against all six major genotypes of hepatitis C, making it one of the most versatile drugs in the arsenal.
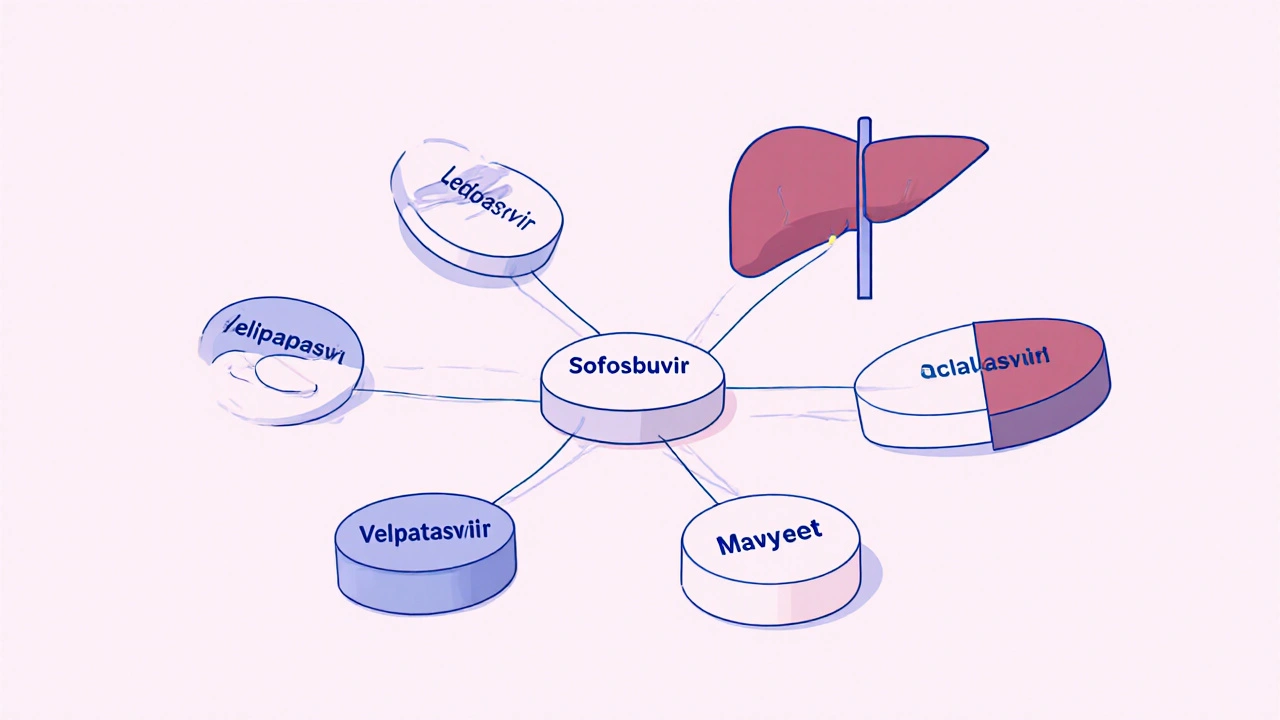
What sets sofosbuvir apart isn’t just its effectiveness - it’s how it’s used. It’s rarely given alone. Instead, it’s combined with other antivirals like ledipasvir, velpatasvir, or voxilaprevir to form fixed-dose combinations. These combos are what actually cure the virus. So when people say "sofosbuvir treatment," they usually mean a combo like Harvoni (sofosbuvir + ledipasvir) or Epclusa (sofosbuvir + velpatasvir).
How do other DAAs stack up?
Since sofosbuvir’s launch, newer drugs have entered the market. Some are better for certain patients. Others are cheaper. A few even work when older treatments failed. Here’s how the main competitors compare:
| Drug Combination | Active Ingredients | Genotype Coverage | Typical Duration | Key Advantage | Key Limitation |
|---|---|---|---|---|---|
| Sofosbuvir + Ledipasvir | Sofosbuvir, Ledipasvir | 1, 4, 5, 6 | 8-12 weeks | High cure rates in genotype 1; once-daily pill | Less effective for genotypes 2 and 3 |
| Sofosbuvir + Velpatasvir | Sofosbuvir, Velpatasvir | All 6 genotypes | 12 weeks | Universal coverage; no need for genotype testing in many cases | Higher cost than older combos |
| Sofosbuvir + Daclatasvir | Sofosbuvir, Daclatasvir | 1, 2, 3, 4 | 12 weeks | Effective for genotype 3; widely available as generics | Requires twice-daily dosing; drug interactions |
| Dasabuvir + Ombitasvir + Paritaprevir + Ritonavir | Dasabuvir, Ombitasvir, Paritaprevir, Ritonavir | 1 | 12 weeks | High efficacy in genotype 1b | Complex dosing; liver toxicity risk; not used in advanced cirrhosis |
| Voxilaprevir + Sofosbuvir + Velpatasvir | Voxilaprevir, Sofosbuvir, Velpatasvir | All 6 genotypes | 12 weeks | Salvage therapy for patients who failed prior DAA treatment | Most expensive option; reserved for retreatment |
Notice something? Sofosbuvir appears in four of the five most common regimens. That’s not an accident. It’s the most reliable engine in the DAA family. Even when newer drugs like glecaprevir or voxilaprevir are added, they’re often paired with sofosbuvir because it’s proven to work with almost anything.
Who benefits most from sofosbuvir combos?
Not every patient needs the same treatment. Your liver health, genotype, and past treatment history matter more than brand names.
- If you have genotype 1 and no cirrhosis, sofosbuvir + ledipasvir (Harvoni) is still a top choice. Cure rates exceed 98%.
- If you have genotype 3 - the hardest to treat - sofosbuvir + daclatasvir is often preferred. Newer combos like Epclusa also work well, but daclatasvir has more real-world data in this group.
- If you’ve failed a prior DAA, the triple combo of voxilaprevir + sofosbuvir + velpatasvir (Vosevi) is the standard. It’s not first-line, but it’s the most reliable rescue therapy.
- If you have advanced cirrhosis or kidney disease, sofosbuvir-based regimens are still safe. Other drugs like glecaprevir/pibrentasvir are avoided here because of liver toxicity risks.
One big win for sofosbuvir combos: they work even if you’ve been infected for decades. A 2024 study in the Lancet Gastroenterology & Hepatology tracked 2,100 patients with long-term hepatitis C. Those treated with sofosbuvir-based regimens had a 96.7% cure rate - regardless of age, weight, or how long they’d had the virus.
Cost and access: The real-world difference
Here’s where things get messy. In the U.S., a 12-week course of Epclusa (sofosbuvir + velpatasvir) can cost over $30,000 without insurance. In the UK, the NHS negotiates bulk prices - so the same treatment costs under £5,000. In low-income countries, generic versions of sofosbuvir are sold for as little as $10 per course.
Generic sofosbuvir is now manufactured in India, Egypt, and Bangladesh under licensing agreements with Gilead. These generics have the same active ingredient, same purity, and same cure rates as the branded version. A 2023 WHO report confirmed that generic sofosbuvir combos achieve the same 95-99% cure rates as the originals.
That’s why sofosbuvir remains the most widely used DAA globally - not because it’s the fanciest, but because it’s the most accessible. In places like Pakistan or Nigeria, where hepatitis C is rampant and healthcare budgets are tight, sofosbuvir-based generics are the only viable option.
Side effects: Are they really better?
One of the biggest myths about DAAs is that they’re side-effect-free. That’s not true. Sofosbuvir combos are better than interferon, but they’re not magic.
The most common side effects are mild: fatigue, headache, nausea. Less than 5% of patients stop treatment because of them. But there are risks.
- Sofosbuvir can lower heart rate when taken with amiodarone - a drug used for irregular heartbeat. This combo can cause dangerous slowing of the heart. Doctors check for this before prescribing.
- Some people on sofosbuvir + velpatasvir report mild increases in liver enzymes. Usually temporary, but monitored.
- Daclatasvir interacts with statins and some seizure medications. If you’re on multiple drugs, your pharmacist should review your list.
Compared to older DAAs like paritaprevir (in Viekira Pak), sofosbuvir combos have far fewer liver toxicity warnings. That’s why they’re now first-choice even for patients with cirrhosis.

What about new drugs without sofosbuvir?
Yes, there are options now that don’t include sofosbuvir. Glecaprevir/pibrentasvir (Mavyret) is one. It’s a once-daily pill that works against all genotypes. It’s also shorter - just 8 weeks for many patients.
But here’s the catch: Mavyret isn’t approved for people with severe kidney disease. It’s also not as well-studied in patients who’ve already failed treatment. And while it’s cheaper than Epclusa in the U.S., it’s still expensive in places without generic access.
Sofosbuvir’s advantage? It’s been studied in over 30,000 patients across 80 countries. Mavyret’s data? Around 4,000. That’s a big difference when you’re choosing a treatment for someone with complex health issues.
Why sofosbuvir still leads in 2025
Is sofosbuvir the absolute best? Maybe not in every single case. But it’s the most reliable, most studied, and most widely available. It’s the foundation. Even when newer drugs are added to the mix, they’re often built around it.
For most people - whether they’re in London, Lagos, or Lahore - sofosbuvir-based regimens are still the smartest first move. They’re simple, safe, and effective. And with generics now standard in most of the world, they’re affordable too.
The future of hepatitis C treatment isn’t about replacing sofosbuvir. It’s about using it better - smarter combinations, shorter courses, and wider access. And right now, no other drug comes close to matching its track record.
Can sofosbuvir cure hepatitis C on its own?
No. Sofosbuvir is never used alone. It’s always combined with another antiviral like ledipasvir, velpatasvir, or daclatasvir. Using it alone increases the risk of the virus becoming resistant. Combination therapy is what makes cure rates over 95% possible.
Is generic sofosbuvir as effective as the brand name?
Yes. Multiple studies, including those by the World Health Organization and the U.S. CDC, confirm that generic sofosbuvir has identical bioavailability, safety, and cure rates to the branded version. The only difference is price - generics cost 95% less in many countries.
How long does it take to cure hepatitis C with sofosbuvir?
Most people take sofosbuvir-based treatment for 8 to 12 weeks. Those with mild liver disease and no prior treatment usually finish in 8 weeks. People with cirrhosis or who’ve failed other treatments typically need 12 weeks. Cure is confirmed with a blood test 12 weeks after finishing treatment.
Can I take sofosbuvir if I have kidney disease?
Yes. Sofosbuvir is one of the few DAAs safe for people with severe kidney disease, including those on dialysis. Other drugs like glecaprevir/pibrentasvir are not recommended in advanced kidney disease. Always check your kidney function before starting treatment.
What happens if sofosbuvir treatment doesn’t work?
If the first course fails, a second-line treatment is available. The most common option is Vosevi (sofosbuvir + velpatasvir + voxilaprevir), which cures over 95% of patients who didn’t respond to earlier DAAs. Treatment failure is rare - under 5% - and usually linked to poor adherence or advanced liver damage.
Do I still need to avoid alcohol after treatment?
Yes. Even after being cured, your liver may still be damaged. Alcohol can cause scarring to return or worsen. Doctors recommend avoiding alcohol completely, especially if you had cirrhosis before treatment. The goal is to protect your liver for life.
What’s next for hepatitis C treatment?
Researchers are already working on even simpler treatments - single-pill, 4-week regimens. Some new drugs in trials don’t even need to be taken daily. But none of them replace sofosbuvir’s role as the backbone of cure.
Right now, the biggest barrier isn’t science. It’s access. Millions still don’t know they’re infected. Others can’t afford treatment, even with generics. The real victory won’t come from a new drug. It’ll come from testing everyone at risk and making sure anyone who needs treatment can get it - no matter where they live.

Written by Felix Greendale
View all posts by: Felix Greendale