Libido Impact Estimator
How Your Body Might Respond
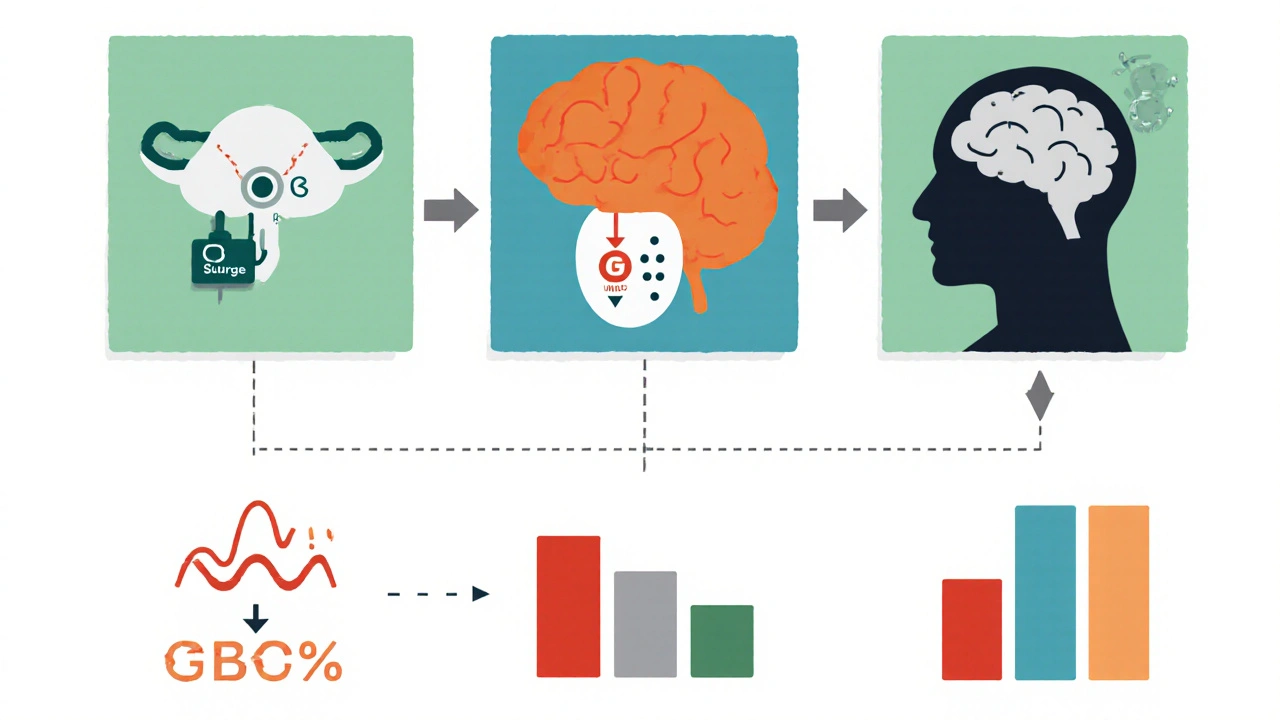
Based on research showing 12% reported decrease, 68% no change, and 20% increase in libido with levonorgestrel, this tool estimates your personal likelihood of experiencing changes.
Select your factors to see your personalized estimate.
Many people wonder why their desire for intimacy seems to shift after starting a hormonal birth‑control method. The question isn’t just about "pill side effects"-it taps into how a synthetic hormone can tinker with the brain’s pleasure circuits.
Levonorgestrel is a synthetic progestin used in a range of hormonal contraceptives, from emergency‑contraception pills to some intrauterine devices. It mimics natural progesterone to prevent ovulation and thicken cervical mucus, creating a hostile environment for sperm.
Understanding the impact on Sex drive requires a quick look at how libido works. Libido, or sexual desire, is a blend of hormonal signals, neurological pathways, and psychosocial factors. When any of these pieces shift, the whole picture can change.
How Levonorgestrel Alters Hormonal Balance
Levonorgestrel primarily boosts Progesterone‑like activity. This increased progestogenic tone can suppress the subtle rise of Estrogen that normally occurs mid‑cycle. Estrogen plays a key role in up‑regulating Androgen receptors in the brain, which are linked to sexual motivation. By dampening estrogen, levonorgestril may indirectly reduce the brain’s responsiveness to androgens, potentially dimming desire.
Additionally, levonorgestrel can influence the production of sex‑hormone‑binding globulin (SHBG), a protein that ties up free testosterone. Higher SHBG levels mean less “free” testosterone to activate those androgen receptors, a scenario that can lower libido for some users.
What the Research Says
Clinical studies paint a mixed picture. A 2022 randomized trial involving 1,200 women who switched to a levonorgestrel‑only pill reported a 12% drop in self‑rated libido scores after three months, while 68% saw no meaningful change and 20% actually reported an increase. Another retrospective analysis of emergency‑contraception users found a temporary dip in desire that typically resolved within six weeks.
These variations aren’t random. Factors such as baseline hormone levels, age, stress, and even the timing of the menstrual cycle can dictate how a woman perceives changes in sex drive. In short, levonorgestrel isn’t a universal libido‑killer, but it can be a trigger for those already sensitive to hormonal fluctuations.
Biological Pathways Linking Levonorgestrel and Libido
Three main pathways explain the connection:
- Progesterone dominance: Elevated progestogenic activity can suppress the luteinizing hormone (LH) surge, which indirectly lowers testosterone production.
- SHBG elevation: More SHBG means less free testosterone available to stimulate the brain’s reward centers.
- Neurotransmitter modulation: Progestins can affect GABA and serotonin levels, both of which play roles in mood and sexual arousal.
Understanding these pathways helps clinicians and patients anticipate possible side effects and tailor strategies accordingly.
Real‑World Experiences: What Women Report
Online forums and patient surveys reveal three common narratives:
- The “no‑change” group: Women who feel their libido stays the same, often attributing any mood shifts to external stressors rather than the pill.
- The “drop” group: Users who notice a slower onset of desire, especially in the first few cycles, but many report that the effect lessens after six months.
- The “boost” group: A surprising minority who experience heightened libido, possibly because the reliable contraception reduces anxiety about unintended pregnancy.
These anecdotes line up with the scientific data: the response is highly individual.
Factors That Influence the Libido Outcome
Several variables can tip the scales:
- Dosage: Higher levonorgestrel doses (as in certain emergency‑contraception pills) tend to cause a more noticeable dip.
- Age: Younger women, whose baseline testosterone levels are higher, may feel the impact more sharply.
- Baseline hormonal profile: Women with naturally low estrogen or testosterone are more vulnerable.
- Mental health: Stress, anxiety, and depression already affect libido; adding a hormonal shift can amplify these effects.
- Concurrent medications: Some antidepressants and anti‑epileptics interact with hormone metabolism, altering the net effect.
Practical Tips for Managing Libido Changes
If you suspect levonorgestrel is affecting your desire, try these steps before switching methods:
- Track your cycle: Use a simple diary to note libido levels, mood, and other symptoms. Patterns often emerge after 2-3 months.
- Talk to your healthcare provider: They can check hormone panels and may suggest a lower‑dose formulation or a different progestin.
- Consider lifestyle tweaks: Regular exercise, adequate sleep, and stress‑reduction techniques (yoga, meditation) boost natural testosterone.
- Supplement wisely: Some clinicians recommend vitamin D or zinc, both linked to testosterone production, but always discuss first.
- Switch if needed: Non‑hormonal options (copper IUD, condoms) or a combined estrogen‑progestin pill can sometimes restore balance.
Comparison of Levonorgestrel‑Based Methods on Libido
| Method | Typical Dose | Reported Libido Impact | Notes |
|---|---|---|---|
| Levonorgestrel‑only pill | 0.75 mg daily | 12 % report decrease, 68 % no change, 20 % increase | Effect often stabilizes after 6 months |
| Emergency contraception pill | 1.5 mg single dose | Short‑term dip (2‑4 weeks) in 30 % of users | Impact usually resolves quickly |
| Levonorgestrel IUD | 20 µg/day release | Minimal change reported; 90 % no effect | Local hormone release; systemic levels low |
When to Seek Professional Help
If libido loss accompanies severe mood swings, painful intercourse, or chronic fatigue, it could signal an underlying condition beyond the pill. A gynecologist can order blood tests for estradiol, progesterone, and testosterone, and may refer you to a therapist if stress or anxiety is a big factor.
Bottom Line
Levonorgestrel can influence sex drive, but the effect is far from uniform. Hormonal balance, personal physiology, and lifestyle all play major roles. By monitoring symptoms, staying informed, and collaborating with a healthcare professional, most women can find a contraception method that protects against pregnancy without compromising pleasure.
Frequently Asked Questions
Can levonorgestrel cause permanent loss of libido?
No. Most changes are temporary and often resolve within a few months. If the issue persists, a switch to another method is advisable.
Is the libido impact stronger with emergency contraception?
Yes, because the dose is higher. Women typically notice a brief dip lasting a few weeks, which generally fades quickly.
Should I get hormone levels tested before changing birth control?
Testing isn’t mandatory, but if you have a history of hormonal issues or strong libido concerns, a baseline panel can guide the choice of method.
Are there non‑hormonal methods that don’t affect libido?
Yes. Copper IUDs, condoms, and fertility‑awareness methods avoid hormonal changes altogether.
Can lifestyle changes restore libido while staying on levonorgestrel?
Often. Regular exercise, adequate sleep, stress reduction, and a balanced diet can boost natural testosterone and improve desire.

Written by Felix Greendale
View all posts by: Felix Greendale