TNF Inhibitor TB Risk Assessment Tool
This tool calculates your TB reactivation risk based on factors discussed in the article. Use it to understand your individual risk and discuss screening needs with your doctor.
Risk Assessment Result
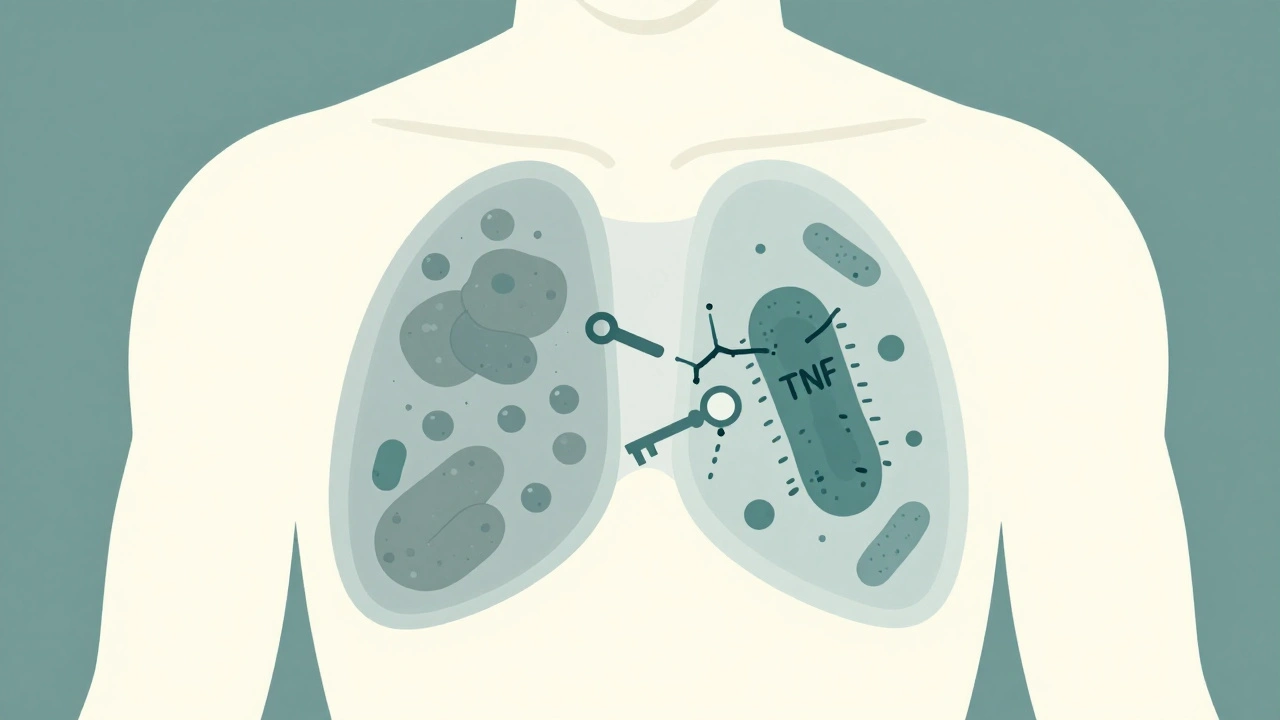
When you start a TNF inhibitor for rheumatoid arthritis, psoriasis, or Crohn’s disease, you’re not just treating inflammation-you’re changing how your body fights infections. The biggest hidden risk? TB reactivation. It’s not rare. It’s not theoretical. It’s a real, documented danger that shows up in clinics across the UK, the US, and beyond-even when patients feel fine and tests come back negative.
Why TNF Inhibitors Put You at Risk for TB
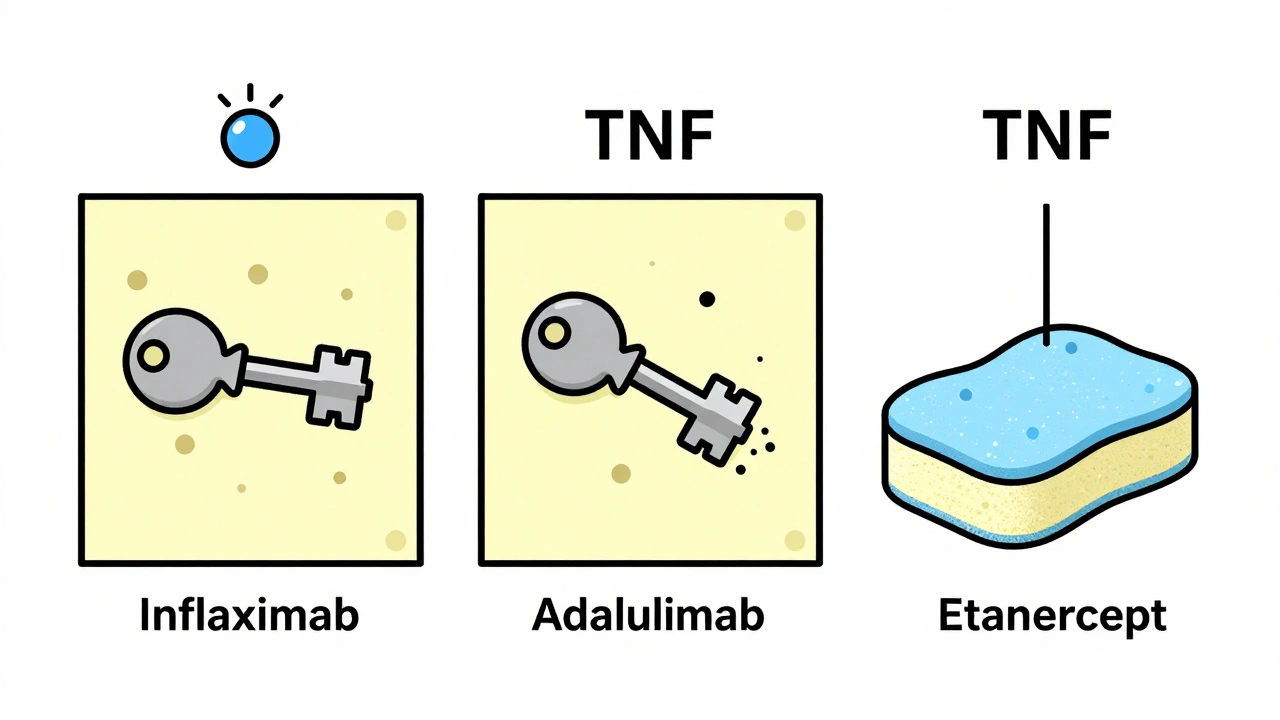
Tumor necrosis factor-alpha (TNF-α) is a protein your immune system uses to build walls around tuberculosis bacteria. These walls, called granulomas, keep the infection locked down, silent, and harmless. That’s latent TB-no symptoms, no spread, no danger… unless you take a TNF inhibitor. Drugs like infliximab and adalimumab block TNF-α completely. They’re powerful antibodies that bind tightly to both free-floating and cell-bound TNF. That’s why they work so well for autoimmune diseases. But it’s also why they break those granuloma walls. Once the wall crumbles, the TB bacteria wake up-and spread. Not all TNF inhibitors are the same. Etanercept works differently. It’s a soluble receptor that soaks up excess TNF but doesn’t stick as hard to the cell-bound version. That’s why it carries a much lower risk. Studies show patients on infliximab or adalimumab are more than three times as likely to reactivate TB compared to those on etanercept.Who’s Most at Risk?
It’s not just about the drug. Where you’re from matters. If you were born or lived for years in a country with high TB rates-India, the Philippines, Nigeria, parts of Eastern Europe, or Latin America-you’re at higher risk. Even if you moved decades ago, your body might still be carrying dormant TB. Age matters too. Older adults are more likely to have had TB exposure in the past. People with diabetes, kidney disease, or who’ve had organ transplants are also at higher risk. And if you’ve been in close contact with someone who has active TB, your risk jumps. Here’s the scary part: 18% of TB cases in TNF inhibitor users happened in people who tested negative before starting treatment. False negatives happen. Screening isn’t perfect.Screening: What You Need to Do Before Starting
Before you get your first TNF inhibitor shot or infusion, you need two tests:- Tuberculin Skin Test (TST): A small shot under the skin. You come back in 48-72 hours to see if there’s a bump. It’s cheap, widely available, but can give false positives if you’ve had the BCG vaccine.
- Interferon-Gamma Release Assay (IGRA): A blood test. It’s more specific-it doesn’t react to BCG. But it’s more expensive and not available everywhere.
What If You Test Positive for Latent TB?
If you have latent TB, you don’t get the TNF inhibitor right away. You treat the TB first. The old standard was 9 months of isoniazid. But that’s hard to stick with. Side effects like liver damage make 32% of people quit. Now, shorter regimens are standard:- 4 months of rifampin
- 3 months of isoniazid plus rifapentine (once weekly)
- 4 months of rifampin plus isoniazid (newly approved in 2024)
Monitoring After You Start
Screening isn’t a one-time thing. You’re still at risk even after you start the drug. The first 3 to 6 months are the most dangerous. Most TB cases show up here. That’s why you need to check in every 3 months for the first year. Your doctor should ask:- Any unexplained fever?
- Night sweats soaking your sheets?
- Weight loss without trying?
- A cough that won’t go away?
The Reality of False Negatives and TB-IRIS
Even with perfect screening, TB can still sneak through. Why? - Recent infection: You were exposed right before your test, and your body hasn’t reacted yet. - Weak immune system: If you’re very sick or on high-dose steroids, your immune system might not respond to the test. - Test limitations: TST can be falsely negative in up to 20% of people with latent TB. And then there’s TB-IRIS-immune reconstitution inflammatory syndrome. It happens when you start TB treatment while still on a TNF inhibitor. Your immune system wakes up, fights the bacteria hard, and causes inflammation. You might feel worse before you feel better: fever spikes, swollen lymph nodes, even worsening lung scans. It’s rare-but serious. About 1 in 8 patients on TNF inhibitors who start TB treatment get TB-IRIS. It often needs steroids to calm down. Your doctor needs to know this is possible.What About Biosimilars?
Biosimilars of adalimumab and infliximab are cheaper now-around $4,500 a month instead of $6,700. But here’s the key: they work the same way. They block TNF-α the same way. The TB risk is identical. Switching to a biosimilar doesn’t lower your risk. It just lowers your bill. Screening and monitoring rules stay the same.

Written by Felix Greendale
View all posts by: Felix Greendale